AAL - Hand Sanitizer Analysis & COVID-19 Swab Testing
We are currently facing a challenging time in which the "Coronavirus" is the dominating topic. We are aware of the increased demands for the manufacturing of health protection products and have great respect for the task of ensuring the continuing supply of these products. Adamson Analytical offers the analysis necessary to move forward with production and some of these analyses can be completed within the same day. With the increased demand for Hand Sanitizers, we have put together the following Information on Time Kill studies which are necessary for proving the efficacy of the product.
Due to the increasing need to ensure the safety of everyone during this time, Adamson Analytical Laboratories has expanded our capabilities to Include COVID-19 Surface Swab Testing. More Information on these analyses is listed below. Feel free to also reach out to Jackie Sirois at jackie.sirois@tentamus.com for any additional questions.
Information on "Time-Kill Test"
Products with antimicrobial properties like disinfectants and sanitizers should be tested for their antimicrobial activities. The ASTM standard method E2315-16 is used to assess the in vitro reduction of a microbial population of test organisms after exposure to a test material. In this test the main parameters that should be varied are the selected microbiological organisms which should be killed, and the exposure time of the product to the microorganisms. The actual number of colonies of living microorganisms remaining after the exposure are then plated on an appropriate agar and then counted after the prescribed incubation period.
It is also strongly recommended that the time kill study is paired with a Minimum Bactericidal Concentration (MBC) or Minimum Inhibitory Concentration (MIC) study as well. These studies help to assess the lowest concentration of an antimicrobial product to kill or inhibit the growth of a specified organism respectively.
The following organisms can be used as representative markers for assessing antimicrobial properties and is recommended for ingredients already classified as GRAS/GRAE (Generally recognized as safe and effective):
Please note that additional organisms can be ordered and included in the study upon request.

COVID-19 Surface Swab Test
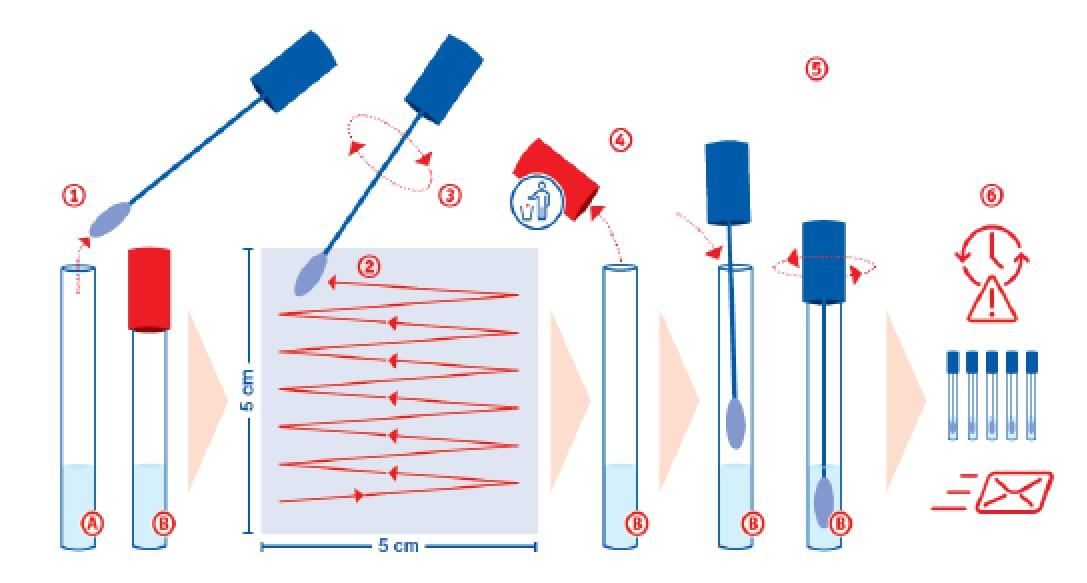
Beginning Wednesday April 8, 2020, we are offering surface swab testing of critical objects for the Coronavirus (SARS-CoV-2).
We provide you with suitable swabs, which you can then use to swab the surfaces of objects including but not limited to:
· Products (Food, Supplies, etc.)
· Mobile phones
· Door handles
· Light Switches
· Handrails
· Keyboards
· Shopping carts
· And many more
These samples will be analyzed in our laboratories by RT-PCR.
With the order, you will receive a COMPLETE swabbing kit that includes everything you need to get started. Just swab your critical areas according to the instructions and seal the vial. We take care of the rest!
Some more specific questions based on frequently asked question will be discussed following.
The analysis is performed using the protocol (“Real-time RT-PCR assays for the detection of SARS-CoV-2”) listed by the WHO.
The result of the detection of coronavirus SARS-CoV-2 / swab (recommended area of 25cm² or 2 in. x 2 in.) RT-PCR Result are expressed as: positive/negative. In the case of positive detection, the test report includes a statement that RNA from coronavirus SARS-CoV-2 has been detected.
The limit of detection of the test system shows 5 -7 RNA copies/µl In tests on surfaces, positive results have been demonstrated with swabs on approx. 10,000 bacteriophages/25cm². (for safety reasons bacteriophages are used as test organisms in the laboratory). This is realistically achievable in a coughing outbreak of an infected person.
No uncertainty of measurement can be determined for qualitative results. Nevertheless, the specificity for the tested microorganisms is 100% - so there are no other viruses that gives the same result.
.jpg)
Please feel free to contact us with any questions or requests for quotations - we are happy to support your business!
.jpg)
Adamson Analytical Laboratories Inc.
220 Crouse Drive, Corona, CA 92879
Office: (951) 549-9657